AP Chem Unit 1 Practice MCQs: A Comprehensive Guide to Success takes a unique approach to the topic, drawing readers into a world of chemistry and engaging them in a memorable and informative way.
This guide provides a thorough overview of the MCQs encountered in AP Chem Unit 1, exploring different types, cognitive skills assessed, and effective preparation strategies. Common pitfalls and how to avoid them are also discussed, along with a set of practice MCQs and a review to reinforce understanding.
MCQ Content Analysis
Multiple-choice questions (MCQs) in AP Chemistry Unit 1 cover a wide range of topics, assessing students’ understanding of fundamental chemical concepts and their ability to apply these concepts to real-world scenarios.
MCQs in this unit can be categorized into several types, including:
Types of MCQs
- Conceptual Understanding:These MCQs test students’ grasp of key chemical concepts, such as atomic structure, bonding, and intermolecular forces.
- Problem Solving:These MCQs require students to apply chemical principles to solve problems involving calculations, data analysis, and experimental design.
- Experimental Design:These MCQs assess students’ ability to design and interpret experiments, as well as their understanding of safety protocols.
- Data Analysis:These MCQs test students’ skills in analyzing and interpreting experimental data, including drawing conclusions and identifying trends.
Cognitive Skills Assessed
The MCQs in AP Chemistry Unit 1 assess a variety of cognitive skills, including:
- Recall:Remembering and applying facts, concepts, and principles.
- Understanding:Comprehending the meaning of chemical concepts and their relationships.
- Application:Using chemical knowledge to solve problems and make predictions.
- Analysis:Breaking down complex chemical information into its components and identifying patterns.
- Evaluation:Judging the validity and relevance of chemical information.
MCQ Preparation Strategies: Ap Chem Unit 1 Practice Mcq
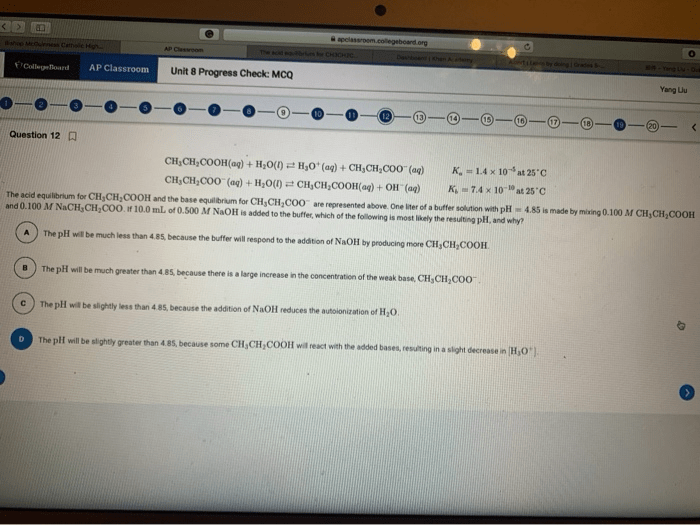
Preparing for AP Chem Unit 1 MCQs requires a strategic approach. Understanding the different types of questions and developing effective time management techniques will enhance your performance.
MCQs in Unit 1 cover a wide range of topics, including stoichiometry, gas laws, and thermodynamics. Each type of question requires a specific approach.
Types of MCQs
Conceptual MCQs:These questions test your understanding of chemical concepts and principles. To answer them effectively, focus on understanding the underlying principles rather than memorizing facts.
Quantitative MCQs:These questions involve calculations and problem-solving. Practice solving problems using the relevant equations and formulas. Show your work to avoid mistakes.
Graphical MCQs:These questions present graphs or diagrams related to chemical concepts. Analyze the graph or diagram carefully and identify the key features to answer the question.
Time Management and Question Selection
Time management is crucial in the MCQ section. Prioritize answering questions that you are confident about first. This will allow you to allocate more time to challenging questions later.
Don’t spend too much time on any single question. If you are stuck, move on and come back to it later if time permits. Guessing randomly is not advisable, but if you must, eliminate the obviously incorrect options first.
Common MCQ Pitfalls

AP Chemistry Unit 1 MCQs pose unique challenges that students must be aware of to succeed. These pitfalls often stem from misunderstandings of concepts, hasty reading, and a lack of effective preparation strategies. Recognizing these pitfalls and implementing strategies to overcome them is crucial for maximizing performance.
Lack of Conceptual Understanding
Many students struggle with MCQs because they lack a solid understanding of the underlying concepts. This can lead to incorrect interpretations of questions and difficulty in applying knowledge to unfamiliar situations. To avoid this pitfall, it is essential to thoroughly review the course material, focusing on developing a deep comprehension of key concepts.
Insufficient Practice
Another common pitfall is insufficient practice with MCQs. Students who are not familiar with the format and style of AP Chem Unit 1 MCQs may find themselves struggling on test day. Regular practice allows students to become comfortable with the question types, time constraints, and the level of difficulty encountered on the actual exam.
Careless Reading
Careless reading can also lead to incorrect answers. Students may misinterpret questions, overlook important details, or make assumptions that are not supported by the text. To avoid this pitfall, it is essential to read each question carefully and thoroughly, paying attention to all the information provided.
MCQ Practice and Review
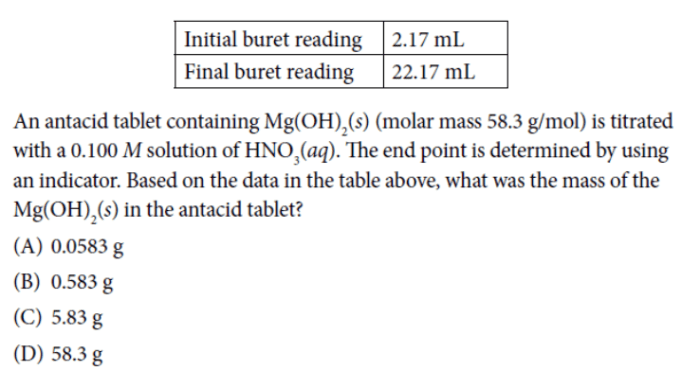
Mastering AP Chemistry Unit 1 concepts requires consistent practice and review. This section provides a comprehensive set of practice MCQs, complete with answer keys and detailed explanations, to reinforce your understanding and prepare you for the exam.
To kick off your AP Chem Unit 1 practice, it’s a good idea to brush up on the basics of organic chemistry. For instance, have you encountered z 1 3 5 tribromo 2 pentene before? Understanding the structure and properties of this compound can help you ace those multiple-choice questions.
So, make sure to include it in your revision plan as you gear up for the AP Chem Unit 1 exam.
Regularly engaging with these practice questions will help you identify areas for improvement, build confidence, and ultimately excel in Unit 1.
Practice MCQs
The following MCQs cover key concepts from AP Chem Unit 1, including atomic structure, periodicity, and chemical bonding.
- Which of the following elements has the highest electronegativity?
- F
- O
- N
- C
- The atomic radius of which element is the largest?
- Li
- Na
- K
- Rb
- Which of the following is a nonpolar covalent bond?
- H-Cl
- C-H
- N-O
- Na-Cl
Answer Keys and Explanations
The answer keys and detailed explanations for the practice MCQs are provided below:
- F has the highest electronegativity due to its small size and high nuclear charge.
- Rb has the largest atomic radius because it has the most energy levels and the outermost electrons are furthest from the nucleus.
- C-H is a nonpolar covalent bond because the electronegativity difference between carbon and hydrogen is small.
Review
After completing the practice MCQs, take some time to review the concepts they cover. Identify any areas where you need further clarification or practice. This review process will help you solidify your understanding and ensure you are well-prepared for the exam.
MCQ Applications

Multiple-choice questions (MCQs) are a valuable tool for assessing student learning in AP Chemistry Unit 1. They allow teachers to quickly and efficiently evaluate students’ understanding of key concepts, theories, and problem-solving skills.
Incorporating MCQs into Classroom
MCQs can be incorporated into the classroom in various ways. They can be used as:
- Formative assessments:Short quizzes or exit tickets to check for student understanding during or after a lesson.
- Summative assessments:Longer tests or exams to assess overall student learning at the end of a unit or chapter.
- Homework assignments:Practice exercises to reinforce concepts and prepare students for assessments.
- Review materials:To help students prepare for upcoming tests or exams.
Tracking Student Progress, Ap chem unit 1 practice mcq
MCQs can also be used to track student progress over time. By administering MCQs at regular intervals, teachers can identify areas where students are struggling and provide targeted support. For example, if a student consistently performs poorly on MCQs related to stoichiometry, the teacher can provide additional instruction and practice in that area.
Key Questions Answered
What is the purpose of this guide?
This guide aims to provide students with a comprehensive understanding of the MCQs encountered in AP Chem Unit 1, helping them prepare effectively for the AP Chemistry exam.
What types of MCQs are covered in this guide?
This guide covers various types of MCQs encountered in AP Chem Unit 1, including multiple-choice, true/false, and short answer questions.
How can I use this guide to prepare for the AP Chemistry exam?
This guide provides effective preparation strategies, practice MCQs, and a review to help students build their knowledge and skills, ultimately enhancing their performance on the AP Chemistry exam.